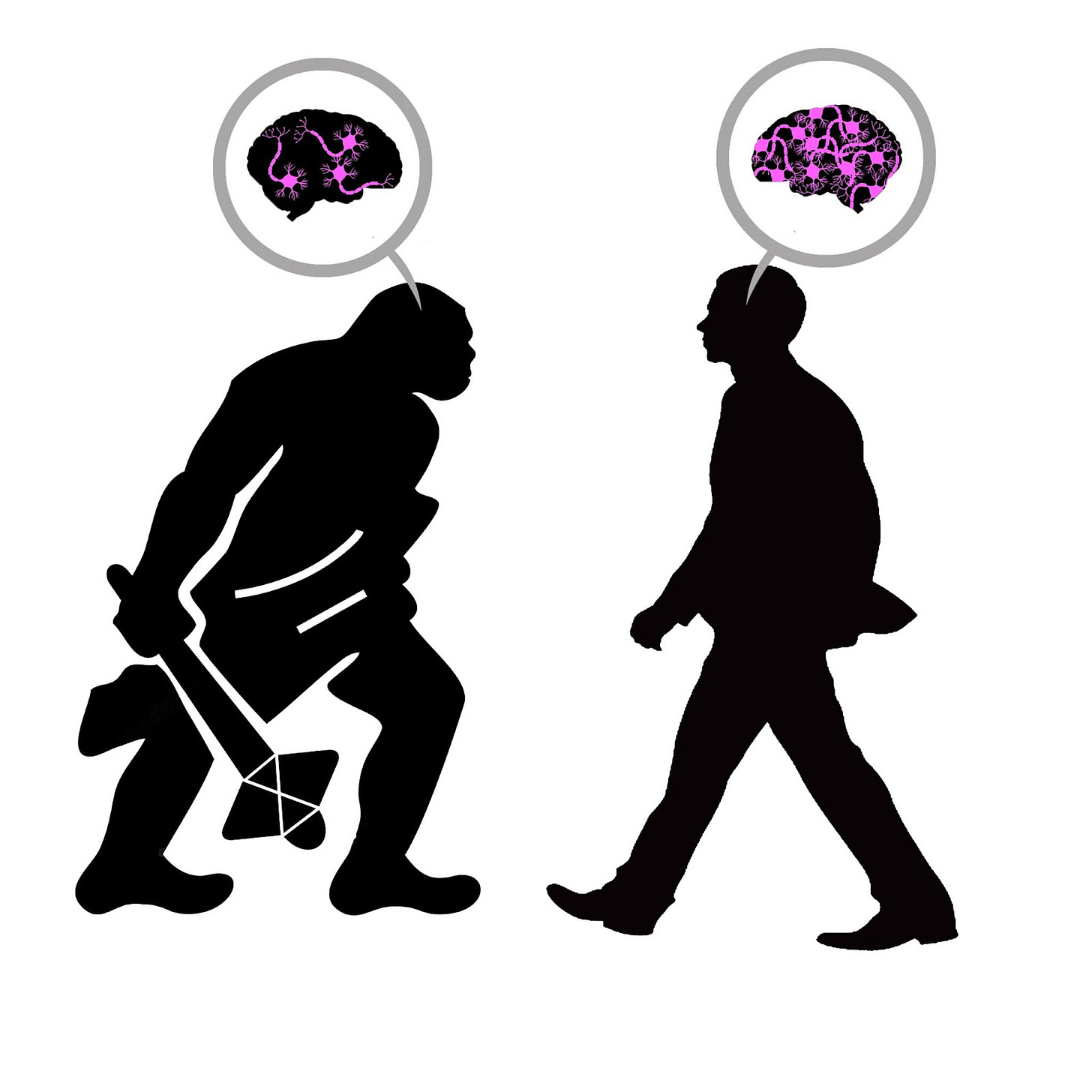
Does a Single Mutation Make Us Smarter Than Neanderthals?
The unique complexity of modern human intelligence is often traced to the neocortex, the (relatively) recent addition to our brains that handles higher-order cognition. However, a study of our Neanderthal relatives raises some interesting questions about this explanation for human exceptionalism, since skull analysis shows that their neocortex was probably just as big as ours. Researchers from the Max Planck Institute for Cell Biology and Genetics offer an explanation, showing that although the modern human neocortex may not be larger, it underwent one lucky mutation that gives us the edge over our ancient comrades.
🧠Article: “Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals” (9/9/2022) - Anneline Pinson et al. “Human TKTL1 Implies Greater Neurogenesis in Frontal Neocortex of Modern Humans than Neanderthals.” Science 377, no. 6611 (September 9, 2022). https://doi.org/10.1126/science.abl6422.
🧠Introduction and Methods:
Though we consider Homo sapiens the most cognitively advanced of the human species, endocranial analysis illustrates that Homo neanderthalensis were probably our equals both in brain and neocortex size. Since the neocortex is considered the root of modern human intelligence, Max Planck researchers wanted to examine whether some other characteristic of this brain region differentiated us from our archaic relatives.
To do this, the team analyzed the genes that play a role in neocortex development. They gave special attention to genes that impact neural progenitor cells, since these cell types have the most direct impact on the number of neurons generated in the cortex. One such gene was hTKTL1, which seems to increase the proliferation of neural progenitors. It was also of interest since it’s one of a handful of genes that is ubiquitous in modern humans but absent from Neanderthals. The team overexpressed these genes in mice, ferrets, and human fetal neocortical tissue to examine the role of hTKTL1 (humanTKTL1) in modern human cortical development and compare it to its Neanderthal counterpart, aTKTL1(archaicTKTL1).
🧠Results and Conclusions:
The Planck team discovered that the hTKTL1 gene increases the abundance of neural progenitor cells called basal radial glia. The Neanderthal variant aTKTL1, which differs from the modern human gene by only a single amino acid substitution, had no effect on the basal radial glia in any of the models. The researchers created a mathematical simulation to show that the higher abundance of these progenitor cells in modern humans would lead to a greater number of cortical neurons, and indeed they found a reduced neuron count in lab-grown human brain tissue that had been made to express aTKTL1 instead of the modern hTKTL1.
Additionally, the researchers found evidence that this single amino acid mutation may have altered the shape of the human neocortex, notably contributing to the expansion of the frontal lobe. This is supported by a higher presence of hTKTL1 mRNA in the frontal lobe compared to the other brain regions examined. The team also notes that the TKTL1 gene may be responsible for giving modern humans their more globular brain and skull shape, while Neanderthals’ appear more elongated.
Even though the modern human brain is no larger than that of our ancient counterparts, it appears that one well-placed amino acid mutation on the TKTL1 gene allowed us to develop cortical neurons more efficiently than any other species on earth. This tiny change deserves responsibility, at least in part, for allowing Homo sapiens to dominate the planet while our relatives died off, and for granting us the complex mental faculties that separate us from the rest of the animal kingdom.
Brief Periods of Food Insecurity Permanently Damage the Brain
In 2021, over 14.5 million U.S. households with children experienced instances of food insecurity. On a global scale, this number shoots into the billions. Food insecurity is a term distinct from starvation and malnutrition, defined by the paper’s authors as “uncertain or limited access to sufficient, nutritionally adequate, and safe food.” Testing the effects of transient periods of insecurity in developing mice revealed permanent impacts on brain development and behavior, highlighting the urgent need to address this global tragedy.
🧠Article: “Transient food insecurity during the juvenile-adolescent period affects adult weight, cognitive flexibility, and dopamine neurobiology” (9/12/2022) - Wan Chen Lin et al. Transient food insecurity during the juvenile-adolescent period affects adult weight, cognitive flexibility, and dopamine neurobiology, Current Biology, Volume 32, Issue 17, 2022, Pages 3690-3703.e5, ISSN 0960-9822, https://doi.org/10.1016/j.cub.2022.06.089.
🧠Introduction and Methods:
Food insecurity is a disturbingly prevalent problem that is linked to devastating cognitive and behavioral outcomes. Uncertain access to food correlates with lower test scores, reduced IQ, longer reaction times, diminished self-control, and difficulties learning. However, since this variable is usually accompanied by other markers of poverty like stress and depression, it’s hard to isolate the impact of feeding history on juvenile and adolescent brain development. To get a clearer picture, UC Berkeley researchers used mouse models to control for any confounding factors. They gave 30 young mice free access to food and gave 25 mice fluctuating and limited access to food before running both groups through a series of behavioral tasks and neurobiological measurements, observing parameters like cognitive flexibility, learning, and dopamine release.
🧠Results and Conclusions:
Interestingly, the effects of food insecurity varied powerfully depending on whether the mouse was male or female. Male food-insecure mice showed a major reduction in cognitive flexibility and probabilistic value predictions, while their adult weights remained the same as their food-abundant counterparts. Food insecure female mice, meanwhile, did not perform worse in the cognitive tasks than the control group, but their adult body weight was significantly higher. The team showed that female mice tend to exhibit a preference for high-fat foods after experiencing food insecurity in the past, while males demonstrate no such behavior. This opens up a line of research questions about the biological mechanisms of the sex difference.
The male mice that had fluctuating feeding schedules were slower at learning to cease unrewarding behaviors, especially when the reward contingencies were more uncertain. This is consistent with less-controlled human studies that correlate a history of transient food deprivation with reduced cognitive flexibility in learning. This may be due in part to changes in dopamine circuits, which were clearly impacted by food insecurity. The team showed that dopamine neurons in the experimental group had weaker glutaminergic inputs, which made them less likely to fire an action potential. They also found that neurons in the dorsal striatum of food insecure mice produced less dopamine compared to the control. Since dopamine plays such a crucial role in facilitating learning and reward sensitivity, these findings are consistent with the behavioral outcomes of the study.
Ultimately, this study controls for the usual confounding factors to present a more definitive picture of the long-term damage caused by food insecurity. We can now say with greater confidence that children who come from food insecure homes suffer major disruptions to their brain development that affect their learning and cognitive flexibility. We also have clear evidence that these changes are mediated by feeding history and not some other correlated marker of poverty. Hopefully this understanding motivates policy changes that will help families receive more regular access to food, sparing children from the permanent repercussions of transient hunger.
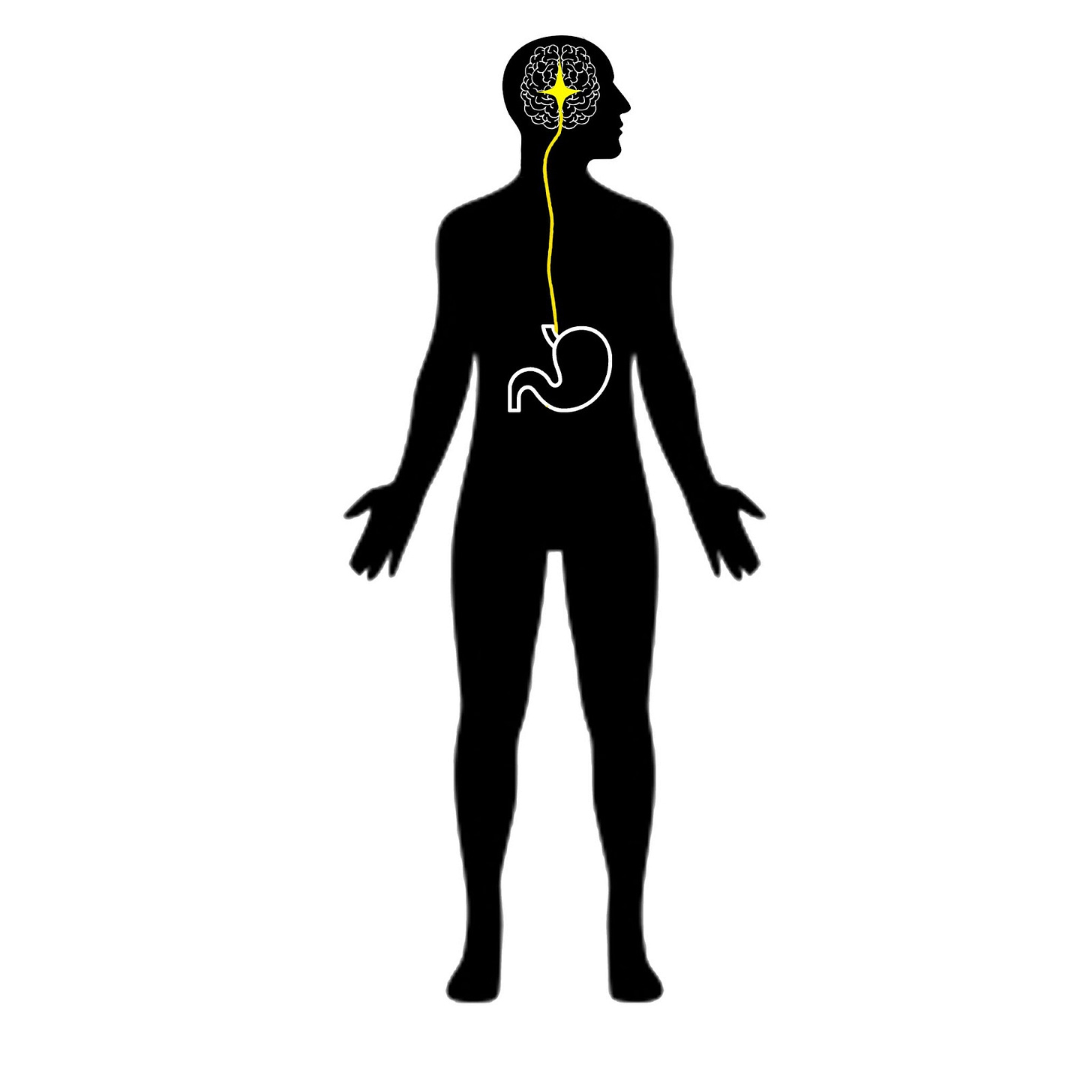
Electrical Stimulation of the Vagus Nerve Helps us Understand Our Stomach
Our stomach and our brain are in constant communication, ensuring that the mind and body are working in step. The nature of this connection is still being explored, but the recent discovery of the gastric brain network indicates that it may play a crucial role in energy expenditure and homeostasis as well as various mental processes like learning and mood. Researchers from the University of Tübingen recently shed some light on the issue by showing that the connection between the brain and stomach can be increased by manipulating the vagus nerve, revealing an illuminating new method for studying this pathway.
🧠Article: “Vagus nerve stimulation increases stomach-brain coupling via a vagal afferent pathway” (9/7/2022) - Sophie J. Müller, Vanessa Teckentrup, Ignacio Rebollo, Manfred Hallschmid, Nils B. Kroemer, Vagus nerve stimulation increases stomach-brain coupling via a vagal afferent pathway, Brain Stimulation, 2022, ISSN 1935-861X,https://doi.org/10.1016/j.brs.2022.08.019.
🧠Introduction and Methods:
The gastric network is a system of brain circuits that align with the rhythms of the stomach and the body’s energy metabolism. The vagus nerve is responsible for signal transmission between the brain and stomach to communicate feelings of hunger and to regulate digestion by causing smooth muscle contractions. In addition to appetite, vagal signaling along the brain-stomach axis has been shown to affect memory, motivation, and mood through the gastric network in the cortex. Disruptions to this network may be associated with various mental disorders, including Parkinson’s disease.
Since the gastric network is a relatively recent discovery, there is an outstanding need for experimental tools to better understand this system. A team of researchers from Tübingen University thought that a process called transcutaneous auricular vagus nerve stimulation (taVNS) could be one such method. taVNS refers to the electrical stimulation of the vagus nerve through the ear, and has been shown to affect cognitive processes that may be related to the brain-stomach axis. The researchers performed taVNS on 31 subjects in conjunction with EEG and fMRI to assess the effect on the brain’s gastric network, and to see if this electrical stimulation affected the participant’s hunger rating.
🧠Results and Conclusions:
Analysis of fMRI data shows that taVNS stimulation increased the connectivity between the brain and stomach, simultaneously activating many of the brain regions involved in the gastric network. Some regions of interest that exhibited connectivity include the solitary nucleus of the brain stem, the dopaminergic midbrain, and the transmodal cortex. The activation of the cortex is in line with the idea that gut signals play a role in subjective experience, and the dopaminergic midbrain response supports the thought that the gut is involved with learning and memory.
The taVNS procedure also produced sensory effects within the brain-stomach axis, altering the participant’s subjective hunger rating with a p-value of less than .001. This suggests potential for a non-invasive treatment of certain eating disorders through taVNS. More broadly, the effects of vagus nerve stimulation on cortical and dopaminergic activity mean that it may prove a useful treatment in a range of mental disorders related to motivation and mood, not just hunger.




Thanks for this. I had missed the food insecurity paper - it will be assigned reading on one of my courses next year. It adds to the disturbing literature on the effects of poverty on the developing brain. And it is so often overlooked. Providing meals in school is an easy long term policy win, but it is an easy target for budget cutting too - as the effects are hidden and may not be seen for years to come.
Super Interesting.... I'm going to add your page to the recommendation list on the brain to mind substack