🧠New Experimental Tool Sheds Light on the Love Hormone
Love, beauty, and interpersonal connection form the core of the humanities, but have long eluded any comprehensive scientific understanding. Though poets may always be more qualified than neurobiologists to speak on love, a newly invented technique for measuring in real-time oxytocin levels lets scientists close the gap just a bit. Oxytocin is a neuropeptide that drives our feelings of romance, arousal, and social bonding, but tools for measuring this crucial chemical have always been clunky at best. A research team out of Osaka University found that a technique using fluorescent proteins may allow us to get a dynamic, real-time understanding of how oxytocin behaves in the brain, which may help clinicians tackle neurological disorders related to the chemical.
🧠Article: “A fluorescent sensor for real-time measurement of extracellular oxytocin dynamics in the brain” (9/24) - Ino, D., Tanaka, Y., Hibino, H. et al. A fluorescent sensor for real-time measurement of extracellular oxytocin dynamics in the brain. Nat Methods (2022). https://doi.org/10.1038/s41592-022-01597-x
🧠Introduction and Methods:
The neuromodulator oxytocin is the biological foundation for many of the feelings that make life worth living. It is mostly recognized for its role in love, intimacy, and social bonding, but it also generates our emotional response to beauty as well as more practical processes like appetite and sensory processing. Despite the chemical’s importance, there is still no widely used method that can monitor oxytocin in real time. Gaining more insight into oxytocin’s pathways is crucial, as disruptions to the chemical are thought to contribute to neurodevelopmental diseases like autism and schizophrenia. Clinicians currently treat these disorders by injecting oxytocin nasally or intravenously, but it's unclear whether these methods are effective since there is no reliable way to track whether the oxytocin is getting to the brain.
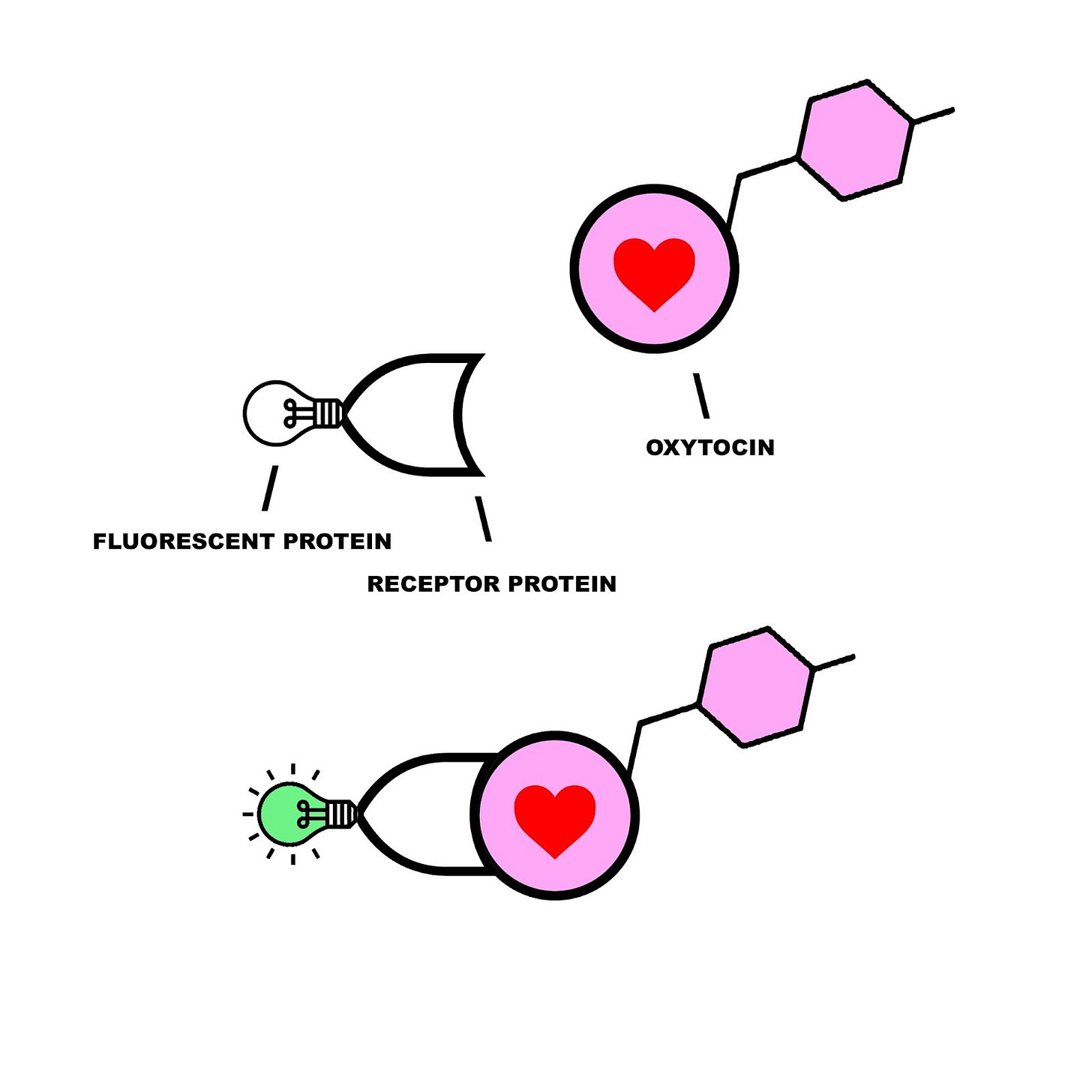
The Osaka University team was inspired by an emerging branch of visualization techniques that had already proven useful in tracking dopamine, acetylcholine, and norepinephrine activity with high temporal resolution. This method involves engineering a compound consisting of a fluorescent protein marker attached to a G-coupled receptor that is sensitive to the target molecule. When the target binds to the G-protein, the fluorescence is activated and can be observed with a strategy called fiber photometry-mediated fluorescence recording.
The team tested proteins from humans, mice, chickens, snakes, and frogs, before settling on a medaka fish protein (meOTR) as the best G-protein for their fluorescent sensor. They then inserted the fluorescent protein MTRIA into meOTR, and began testing whether it could measure exogenous and endogenous oxytocin in living mice. If successful, they would also use this technique to see how factors like aging, anesthesia, and food deprivation affect oxytocin levels.
🧠Results and Conclusions:
The team found that any level of brain-injected oxytocin exceeding 0.2 micrograms caused a major increase in measurable fluorescent activity, meaning that their method seemed an effective sensing tool. Importantly, this 0.2 microgram threshold is similar to the level required to trigger behavioral effects in animals.
After confirming their method’s effectiveness at measuring exogenously administered oxytocin in live mice, the team wanted to test if this was true for oxytocin produced by the mouse brain itself. To do so, researchers tracked oxytocin levels in the anterior olfactory nucleus as mice went freely about their daily lives. Not only did the team find that their method produced clear measurements, but they also made the key discovery that oxytocin levels oscillated regularly on a two-hour cycle. The team thinks that this may be related to feeding rhythms, but further research on this newfound pattern is needed.
After confirming that their sensing method could detect endogenous and exogenous oxytocin with high temporal resolution, the team put their strategy to practical use by testing whether common clinical injection methods actually affected the brain. They discovered that even large doses of oxytocin injected through the nose or into the abdominal wall do not increase oxytocin in the brain regions they examined, which means that current medical treatments may need to be revised. They also used their technique to discover that aging, anesthesia, and food deprivation all disrupt oxytocin levels, meaning that these factors must be considered in future experiments that involve oxytocin measurement.
The team’s fluorescent oxytocin sensor is a significant step towards understanding one of our most vital neuromodulators, and has already proven useful in refining clinical methods for oxytocin administration and in illuminating oxytocin patterns in healthy and impaired individuals. With further study, scientists may employ similar techniques to develop schizophrenia and autism treatments and to deepen our neurochemical understanding of love and bonding in general.
🧠Do Hierarchies Reduce Empathy?
Some of the most brutal tragedies in human history stemmed from the diffusion of responsibility across a chain of command, separating the person giving the orders from the individuals carrying them out. A brain imaging study from the Royal Netherlands Academy (KNAW) sought to examine the neurological basis of this phenomenon, examining how brain regions related to empathy and agency acted differently when violent decisions were made at varying positions in a hierarchy.
🧠Article: “Commanding or being a simple intermediary: how does it affect moral behavior and related brain mechanisms?” (9/29/22) - Caspar, E. A., Ioumpa, K., Arnaldo, I., Di Angelis, L., Gazzola, V., & Keysers, C. (2022). Commanding or being a simple intermediary: How does it affect moral behavior and related brain mechanisms? Eneuro. https://doi.org/10.1523/eneuro.0508-21.2022
🧠Introduction and Methods:
The worst examples of human cruelty occur when responsibility is split across a hierarchy. When a subordinate is separated from the decision-making process and a superior is separated from the decision’s outcome, people seem capable of greater evils than any one person could commit on their own. The famous Milgram Shock Experiment shows that this disconnect promotes ruthless behavior, but no previous study has examined the underlying neural mechanisms that allow us to act more cruelly as part of a hierarchy. The researchers also wanted to understand how adding robots to the bottom of this hierarchy affects human empathy. In an era when drones, missiles, and robots promise to be the future of warfare, it is crucial to understand how this added level of distance affects human morality and violence.
Psychological studies have hypothesized that hierarchies affect our willingness to harm by reducing our sense of agency and our empathy for pain. Our sense of agency determines the extent to which we feel responsible for the impact of our actions, and it has been traced to specific brain regions including the striatum, basal ganglia, and frontal motor areas. Empathy for pain involves processing another’s pain within our own pain system, and registers as an activation of the brain’s ‘pain matrix’ which involves the anterior cingulate cortex and the anterior insula.
The study involved 70 participants that were put into pairs. One participant played the role of the victim, while the other was either a ‘commander’ or an ‘intermediary.’ As a commander, they could choose whether or not to order a painful shock to the victim to receive a monetary reward (the action itself was carried out by either an automated agent or another human). As an intermediary, the researcher gave the orders and the participant simply carried them out. The first leg of the study used fMRI to measure empathetic brain activity as the participant witnessed the victim receiving the shock. The second branch of the study used EEG to measure both empathy and sense of agency in the same scenario.
🧠Results and Conclusions:
After measuring brain activation in commanders and intermediaries using fMRI, the team found that both groups displayed similar levels of pain empathy. The EEG study, however, showed that there was more empathetic activation in commanders than in intermediaries, but only when the commander gave orders to a robotic agent instead of a human. This supports the idea that empathy is reduced when people feel like they are sharing responsibility for a decision with another person. When they give commands to an inanimate agent, they cannot offload responsibility and feel more connected to the outcome of their decision.
When it came to sense of agency, there were significant differences in brain imaging data between commanders and intermediaries. Commanders exhibited increased activity in the brain regions corresponding with feelings of responsibility. Participants also subjectively reported higher feelings of responsibility when in the commander position, corroborating the imaging results.
Though we have substantial psychological and historical evidence showing that humans are more prone to cruel behaviors within a hierarchy, no study had ever examined a concrete neural basis for this behavior. By revealing a link between harmful behavior and changes in activation in brain regions that control responsibility and empathy, the KNAW researchers showed how diffusing responsibility can produce inhumane results when unchecked. Since commanders showed more empathy for their victim when ordering a robot instead of another human, this may be one way that we can limit our capacity for cruelty in certain hierarchies.
🧠 Is Obesity a Neurodevelopmental Disorder?
Although obesity is now disturbingly common across the globe, research on the crucial neurobiological components of this affliction remains murky. Baylor University researchers wanted to see if obesity could be partially traced to lasting changes in gene expression that occur in the hypothalamic arcuate nucleus during a critical period shortly after birth. Environmental factors during this window seem to affect neural development in a way that permanently impacts hunger, metabolism, and physical activity, causing a lifelong increase in BMI.
🧠Article: “Sex-specific epigenetic development in the mouse hypothalamic arcuate nucleus pinpoints human genomic regions associated with body mass index” (9/28/2022) - MacKay H, Gunasekara CJ, Yam KY, Srisai D, Yalamanchili HK, Li Y, Chen R, Coarfa C, Waterland RA. Sex-specific epigenetic development in the mouse hypothalamic arcuate nucleus pinpoints human genomic regions associated with body mass index. Sci Adv. 2022 Sep 30;8(39):eabo3991. doi: 10.1126/sciadv.abo3991. Epub 2022 Sep 28. PMID: 36170368.
🧠Introduction and Methods:
Obesity has quickly burgeoned into a dire global health epidemic, now affecting over 2 billion individuals worldwide. Though it’s tempting to view this affliction from the lens of physical health alone, it’s becoming increasingly clear that the biology of the brain plays a crucial role in predicting lifelong obesity. Certain periods of brain development affect critical factors such as energy metabolism and propensity for physical exercise which correlate highly with BMI. When the brain undergoes abnormal development in these periods it often results in obesity, leading researchers to propose that it may best be understood as a neurodevelopmental disease.
The researchers felt that epigenetic factors (which describe how our environment changes our genetic expression) were chiefly responsible for the neural roots of obesity, especially when it came to sex differences and the suckling critical period. They hypothesized that the hypothalamic arcuate nucleus (ARH), a brain region involved in energy metabolism, underwent crucial obesity-related epigenetic development in the window immediately following birth. To study this, the team examined the genetic profiles of neurons and glial cells in the mouse ARH during this critical period to uncover clues that could link neural development to BMI. They dissected the mouse brains at strategic ages and used whole-genome bisulfite sequencing and RNA sequencing to see which DNA segments in ARH cells were being expressed. They segmented their results to make comparisons between males and females, as well as between different cell types.
🧠Results and Conclusions:
The team’s analysis of the ARH genome found that the suckling period following birth is a crucial critical window for establishing a healthy energy metabolism later in life. Disrupted environmental factors during this time can cause certain genes in the hypothalamus to inactivate permanently, leading to a much higher risk for obesity. They also found a series of previously undiscovered hypothalamic sex differences that affect BMI and appetite. It appears that during this critical window, females become more responsive to leptin which is a hormone that inhibits hunger and gives cues about energy levels. Females also showed greater expression of proopiomelanocortin (POMC), which is a gene that similarly mediates appetite. When POMC levels are deficient in mice or humans, the result is an insatiable appetite which typically leads to an unhealthy weight.
Aside from from sex differences, the team found that nutritional deficiencies during this postnatal critical period may permanently alter genetic expression in the ARH through Lhx, Dlx, and Sox transcription factors. These proteins can “turn off” certain genes by blocking the transcription of DNA into RNA. They found that the genetic regions that had previously been linked to heritable obesity were highly affected by epigenetic transcription factors in this critical window, tracing the root of the disorder back to neurodevelopment as opposed to raw genetics or psychology. Importantly, this means that optimizing environmental factors such as nutrition in this brief time window may drastically reduce the odds of obesity later in life.
The team’s data also indicates that neuroepigentic development is highly conserved between humans and mice, which not only means that the results of this study can be extrapolated to humans but also that researchers can more confidently use mouse models to study epigenetics in the brain moving forward.
Ultimately, this study provides evidence that environmental factors in early life can permanently impact the brain’s genetic expression in a way that leads to lifelong obesity. Genes in the hypothalamic arcuate nucleus that have been linked to obesity were shown, by comparing the genomes and RNA profiles of an array of mice, to be highly sensitive to epigenetic changes in the postnatal critical period. This deepened understanding of obesity’s neural mechanisms may inform strategies to curb this public health crisis moving forward.
If you found value in our zero-cost information, you can support the newsletter by sharing it with a friend or by using the following links to purchase one of the curated neuroscience books we chose to review, Livewired by David Eagleman or The Body Keeps the Score by Bessel van der Kolk. Thanks for reading!






A great read, thanks. The effects of oxytocin might be a little more complicated than "love," however...
https://www.ted.com/talks/molly_crockett_beware_neuro_bunk?language=en
Really enjoyed the discussion about oxytocin! I've heard of this (admittedly strange) phenomenon called the "birthgasm", where pregnant women who deliver a baby feel a sensation similar to that felt during an orgasm because of oxytocin's role. I've been learning about epigenetics as well. Fascinating stuff.